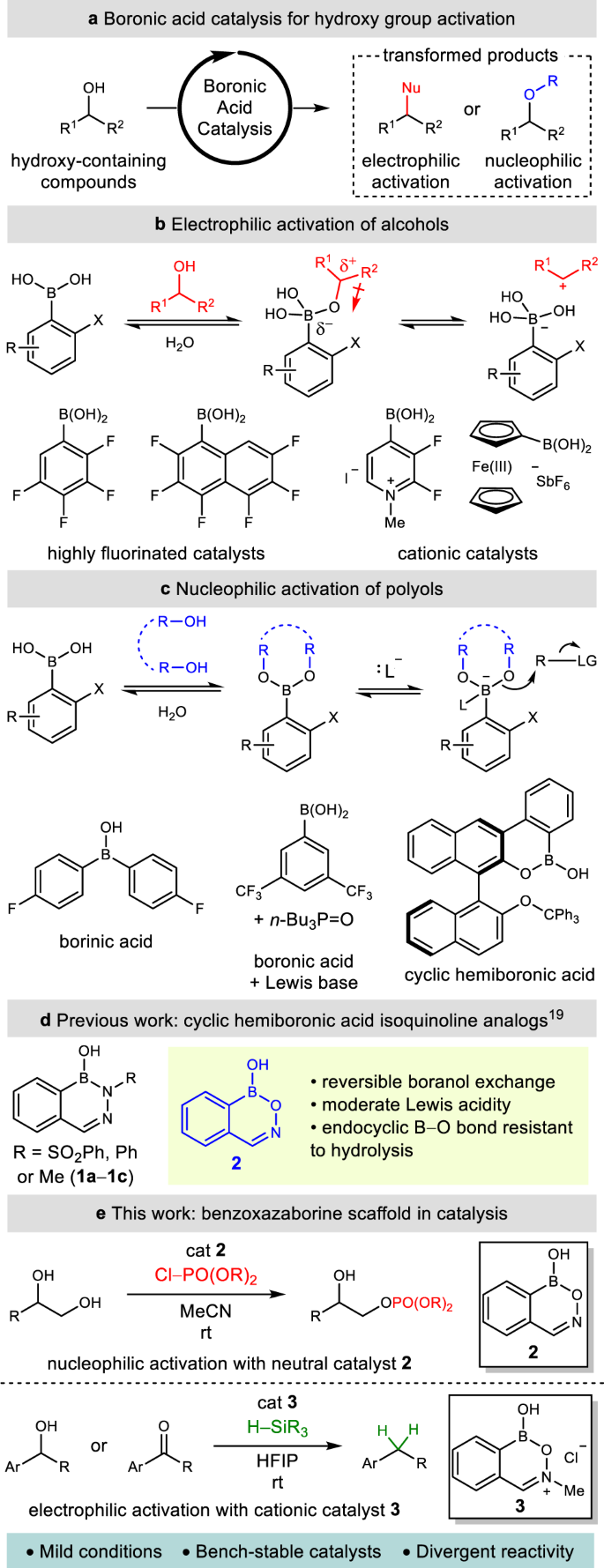
Direct nucleophilic and electrophilic activation of alcohols using a unified boron-based organocatalyst scaffold | Nature Communications

Protodeboronation of Heteroaromatic, Vinyl, and Cyclopropyl Boronic Acids: pH–Rate Profiles, Autocatalysis, and Disproportionation | Journal of the American Chemical Society

Streamlined Process for the Chemical Synthesis of RNA Using 2′-O-Thionocarbamate-Protected Nucleoside Phosphoramidites in the Solid Phase | Journal of the American Chemical Society
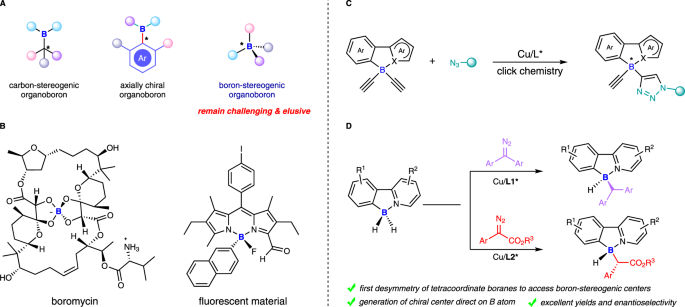
Construction of boron-stereogenic compounds via enantioselective Cu-catalyzed desymmetric B–H bond insertion reaction | Nature Communications

Evidence for Borylene Carbonyl (LHB═C═O) and Base-Stabilized (LHB═O) and Base-Free Oxoborane (RB≡O) Intermediates in the Reactions of Diborenes with CO2 | Journal of the American Chemical Society

Hydrogen peroxide-activatable antioxidant prodrug as a targeted therapeutic agent for ischemia-reperfusion injury | Scientific Reports
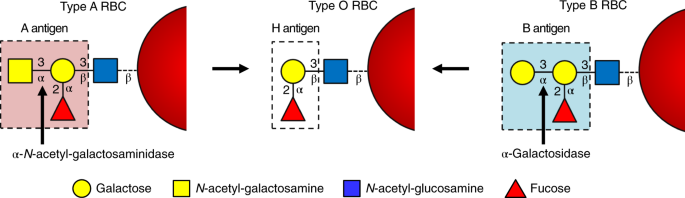
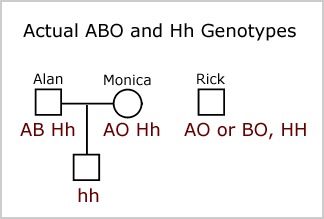
An enzymatic pathway in the human gut microbiome that converts A to universal O type blood | Nature Microbiology
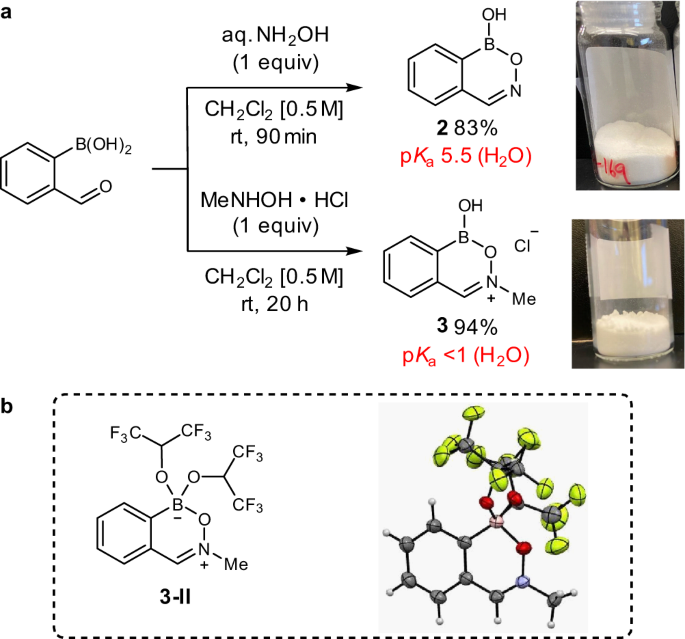
Direct nucleophilic and electrophilic activation of alcohols using a unified boron-based organocatalyst scaffold | Nature Communications

Tri(1-adamantyl)phosphine: Expanding the Boundary of Electron-Releasing Character Available to Organophosphorus Compounds | Journal of the American Chemical Society